Groundwater Quality
Groundwater quality in Utah is good in most places. Exceptions occur where groundwater travels slowly through an aquifer that consists of chemically reactive minerals such as halite (salt) or gypsum, which may dissolve into the groundwater. Mineral dissolution increases the total dissolved solids (TDS) concentration and may add constituents that limit or preclude its use (for example, high salinity, sulfate, or arsenic). The type of geologic materials in a groundwater basin and the length of time groundwater is in contact with those materials are fundamental controls on water chemistry.
Dissolved Solids
Groundwater in the basin- and valley-fill aquifers is typically fresh, dominated by calcium, magnesium, and bicarbonate (HCO3–) ions, and generally has dissolved-solids concentrations less than 1,000 milligrams per liter (mg/L). Groundwater having less than 250 mg/L TDS concentration is typically located in recharge areas along valley or basin margins and in high mountainous areas. Lower parts of valleys/basins typically contain more saline (salty) water.
- Groundwater with high TDS concentrations, especially calcium and sulfate, exists in some areas having rocks abundant in gypsum or salt, such as in wells downgradient from the salt-rich Arapien Shale in Sanpete Valley.
- Groundwater with high TDS concentrations, especially sodium and chloride, exists near playa areas and saline lakes, such as in wells located near the shore of Great Salt Lake.
Water quality often declines over time in areas where groundwater pumping for irrigation lowers the water table and recharge comes from water recycled through the soil, such as the Sevier Desert, Pahvant Valley, and the Beryl-Enterprise area. Sandstone aquifers generally have TDS concentrations less than 1,000 mg/L.
Groundwater Chemistry and Environmental Tracers
The water chemistry from wells, springs, and streams in different locations and at different well depths, when viewed with other physical data, can help scientists infer flow paths and residence time of groundwater and interactions with surface water.
The proportion and amount of dissolved ions in groundwater, often visualized using Piper diagrams, can reveal clues about how much mixing occurs between aquifers and which rocks have the most influence on groundwater chemistry.
Environmental tracers are naturally occurring constituents, man-made chemicals, or isotopes that can indicate water sources and flow processes such as recharge, flow rate, geologic subsurface interactions, residence times, and mixing between sources. Data from environmental tracers coupled with solute chemistry can help identify groundwater provenance (origin) and surface water/groundwater interaction. Ideal tracers have well defined input sources and input histories, are inert (no reactions) or geochemically conservative (limited reactions), have transport mechanisms identical to water, and are detected precisely and economically. Tracers commonly used by UGS scientists include stable isotopes of water (oxygen-18 [δ18O] and deuterium [δ2H]), tritium (3H), noble gases (helium, neon, argon, krypton, and xenon), and radiocarbon (carbon-14 [14C] and carbon-13 [δ13C]).
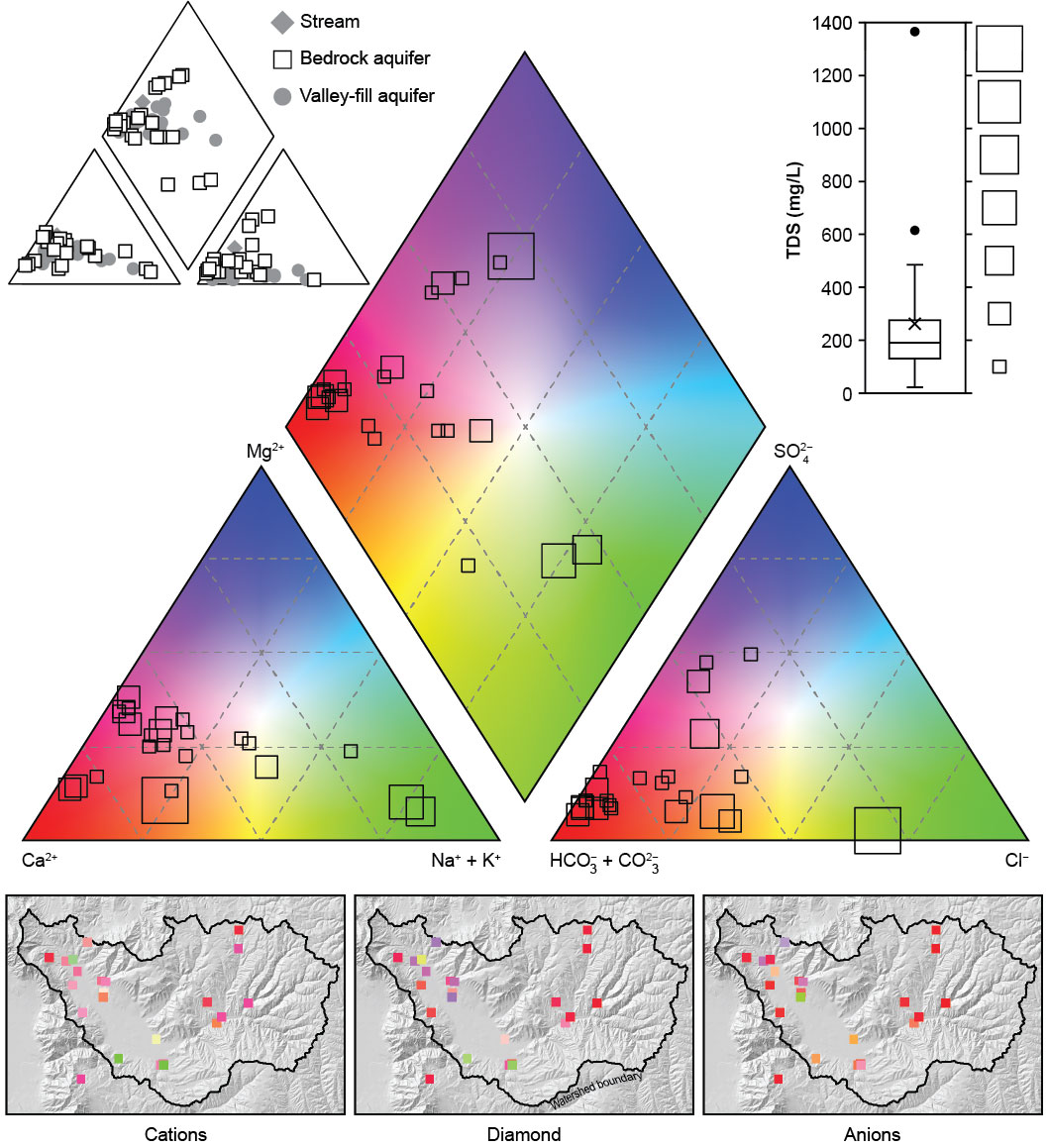
FIGURE: Piper diagram of water chemistry in bedrock wells in Ogden Valley, Weber County, Utah. The location of symbols in the triangles shows the relative proportion of cations and anions in samples, whereas the size of the symbols is proportional to the amount of total dissolved solids (TDS) in the samples. The maps below the Piper diagram show where each of the samples are located, allowing the viewer to see where groundwater may be influenced by rock type. For example, limestone likely influences wells located at red squares (calcium-bicarbonate dominant water) and halite may be impacting green square wells (sodium-chloride dominant water). Comparing this Piper diagram of bedrock groundwater chemistry to similar diagrams of streams or the valley-fill aquifer wells can show the location of bedrock aquifer discharge to the valley fill or streams.
Groundwater Quality Classification
Many communities in Utah are experiencing population growth. Many rural areas in Utah depend on groundwater as their primary source of water within valley-fill aquifers, and with increasing population growth, some communities are opting to classify their local aquifer. The UGS has been involved with 10 hydrogeologic studies in Utah to help communities gather the necessary hydrogeologic data required to formally classify aquifers, and thereby provide a measure of protection to their groundwater resources.
Under Utah’s Administrative Rules for Ground Water Quality Protection, groundwater quality classes are based on total-dissolved-solids (TDS) concentrations as shown in the table below. Two other classes, IB and IC, are not based on groundwater chemistry.
Groundwater quality classes under the Utah Water Quality Board’s total-dissolved-solids- (TDS) based classification system
(modified from Utah Division of Water Quality, 1998)
|
Groundwater Quality Class |
TDS Concentration |
Beneficial Use |
| Class IA/IB1/IC2 | Less than 500 mg/L3 | Pristine/Irreplaceable/Ecologically Important |
| Class II | 500 to less than 3000 mg/L | Drinking Water4 |
| Class III | 3000 to less than 10,000 mg/L | Limited Use5 |
| Class IV | 10,000 mg/L and greater | Saline6 |
1Irreplaceable groundwater (Class IB) is a source of water for a community public drinking-water system for which no other reliable supply of comparable quality and quantity is available due to economic or institutional constraints; it is a groundwater quality class that is not based on TDS.
2Ecologically Important groundwater (Class IC) is a source of ground-water discharge important to the continued existence of wildlife habitat; it is a groundwater quality class that is not based on TDS.
3For concentrations less than 7000 mg/L, mg/L is about equal to parts per million (ppm).
4Water having TDS concentrations in the upper range of this class must generally undergo some treatment before being used as drinking water.
5Generally used for industrial purposes.
6May have economic value as brine.